CSIR-NET LIFE SCIENCE NOTE FOR UNIT-1 -A. Structure of atoms, molecules and chemical bonds.
A. Structure of atoms, molecules and chemical bonds.
1.ATOM-
Atoms consist of electrons surrounding a nucleus that contains protons and neutrons.
Neutrons are neutral, but protons and electrons are electrically charged. Protons have a relative charge of +1, while electrons have a relative charge of -1.
The number of protons in an atom is called its atomic number. In the periodic table atoms are arranged in atomic number order.
Electrons are arranged in energy levels or shells, and different energy levels can hold different numbers of electrons. The electronic structure of an atom is a description of how the electrons are arranged, which can be shown in a diagram or by numbers. There is a link between the position of an element in the periodic table and its electronic structure.
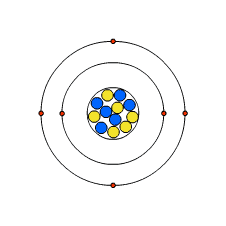
The structure of an atom
Although the word 'atom' comes from the Greek for indivisible, we now know that atoms are not the smallest particles of matter. Atoms are made from smaller subatomic particles.
Both protons and electrons have an electrical charge. Both have the same size of electrical charge, but the proton is positive and the electron negative. The neutron is neutral.
The electrical charge of particles
| particle | relative charge |
|---|---|
| proton | +1 |
| neutron | 0 |
| electron | -1 |
he total number of electrons in an atom is always the same as the number of protons in the nucleus. This means atoms have no overall electrical charge.
The number of protons in an atom is called its atomic number - also called the proton number. Atoms are arranged in the periodic table in order of increasing atomic number
Energy levels and shells
Electrons are arranged in different shells around the nucleus. The innermost shell - or lowest energy level - is filled first. Each succeeding shell can only hold a certain number of electrons before it becomes full. The innermost shell can hold a maximum of two electrons, the second shell a maximum of eight, and so on. The table gives the maximum capacity of the first three shells.
Maximum capacity of the first three shells
| energy level or shell | maximum number of electrons |
|---|---|
| first | 2 |
| second | 8 |
| third | 8 |
A lithium atom, for example, has three electrons. Two are in the first energy level, and one in the second.
A carbon atom has six electrons. Two are in the first energy level, and four in the second energy level.
A calcium atom has 20 electrons. Two are in the first energy level, and eight in the second energy level, eight in the third energy level and two in the fourth energy level.
Electronic structure 1
The electronic structure of an atom is a description of how the electrons are arranged. For your exam, you need to be able to describe the electronic structure of the first 20 elements in the periodic table. You may need to re-visit the section in AQA GCSE Science on the periodic table for this.
The first 20 elements in the periodic table run from hydrogen to calcium. Their electronic structures can be shown either as diagrams or numbers. You need to know how to do both.
Take lithium, for example. The drawing shows each energy level as a circle around the nucleus, with each electron represented by a dot. In the exam, do not worry about colouring in the electrons. Just make them clear and ensure they are in the right place. Sometimes you will be asked to use a cross rather than a dot. The numerical method is to write the chemical symbol (Li ) followed by the number of electrons in each energy level, innermost first, Li 2,1.
Electronic structure of lithium
| Element | Numeric format | Electrons | Periodic table group |
|---|---|---|---|
| Li 2,1 |
Lithium atoms have three electrons. Two of these fit into the first energy level, with the third in the second energy level.
|
Group 1
|
Electronic structure 2
Below are some more electronic structures. Remember - you need to learn the electronic structures of the first 20 elements.
The number of electrons in the highest occupied energy level of each atom is the same as the element's group number.
Electronic structures of elements
| Element | Numeric format | Electrons | Periodic table group |
|---|---|---|---|
| F 2,7 |
Fluorine atoms have nine electrons. Two of these fit into the first energy level. The remaining seven fit into the second energy level.
|
Group 7
| |
| Ne 2,8 |
Neon atoms have ten electrons. Two of these fit into the first energy level. The remaining eight electrons fit into the second energy level. Because its highest occupied energy level is full, neon is stable and unreactive.
|
Group 0 - that is, the eighth group
| |
| Na 2,8,1 |
Sodium atoms have 11 electrons. Two of these fit into the first energy level, eight into the second energy level. The last one fits into the third energy level.
|
Group 1
| |
| Ca 2,8,8,2 |
Calcium atoms have 20 electrons. Two of these fit into the first energy level, eight into the second energy level, another eight into the third energy level. The last two fit into the fourth energy level.
|
Group 2
|
2.MOLECULE-
A molecule is the smallest particle in a chemical element or compound that has the chemical properties of that element or compound. Molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms. The atoms of certain elements readily bond with other atoms to form molecules. Examples of such elements are oxygen and chlorine. The atoms of some elements do not easily bond with other atoms. Examples are neon and argon.
Molecules can vary greatly in size and complexity. The element helium is a one-atom molecule. Some molecules consist of two atoms of the same element. For example, O2 is the oxygen molecule most commonly found in the earth's atmosphere; it has two atoms of oxygen. However, under certain circumstances, oxygen atoms bond into triplets (O3), forming a molecule known as ozone. Other familiar molecules include water, consisting of two hydrogen atoms and one oxygen atom (H2O), carbon dioxide, consisting of one carbon atom bonded to two oxygen atoms (CO2), and sulfuric acid, consisting of two hydrogen atoms, one sulfur atom, and four oxygen atoms (H2 SO4).
Some molecules, notably certain proteins, contain hundreds or even thousands of atoms that join together in chains that can attain considerable lengths. Liquids containing such molecules sometimes behave strangely. For example, a liquid may continue to flow out of a flask from which some of it has been poured, even after the flask is returned to an upright position
Molecules are always in motion. In solids and liquids, they are packed tightly together. In a solid, the motion of the molecules can be likened to rapid vibration. In a liquid, the molecules can move freely among each other, in a sort of slithering fashion. In a gas, the density of molecules is generally less than in a liquid or solid of the same chemical compound, and they move even more freely than in a liquid. For a specific compound in a given state (solid, liquid, or gas), the speed of molecular motion increases as the absolute temperature increases.
3.CHEMICAL BOND-
Atoms form bonds with other atoms in order to have a full outer shell of electrons like the noble gases. If an atom has too few or too many valence electrons it will have to gain, lose, or share those outer electrons with another atom in order to become “happy” or in chemistry terms, more stable. There are many types of chemical bonds that can form, however the 3 main types are: ionic, covalent, and metallic bonds. You must become familiar with how they work and the differences between the 3 types.There are many types of chemical bonds that can form, however the 3 main types are: ionic, covalent, and metallic bonds. You must become familiar with how they work and the differences between the 3 types.
I. Ionic bonding: Model 1 is a description of what chemists call ionic bonding. Ionic bonding occurs strictly between metal and nonmetal atoms. In ionic bonding some of the valence electrons of a metal atom are transferred to a nonmetal atom so that each atom ends up with a noble gas configuration. Usually one, two, or three electrons are transferred from one atom to another. This transfer of an electron causes the metal atom to have a net positive charge (+) and the nonmetal atom to have a net negative charge (-). The individual atoms in ionic solids are referred to as ions because of their charges. These opposite charges are attracted to one another. On the right is a drawing of a chunk of salt, NaCl, a very common ionic substance. Notice how the sodium and chloride ions alternate throughout the structure. The positive and negative ions alternating in three dimensions make the solid quite strong because of their strong attractions to one another. The sodium ion is written Na+ and the chloride ion is written Cl-. When ionic solids are placed in contact with water, they dissolve. They remain ions, with charges, but now the individual ions are surrounded by water molecules and distributed throughout the water. Once dissolved, ionic compounds will conduct electricity.
II. Covalent Bonding: Model 2a represents bonding that is referred to by chemists as covalent bonding. The valence electrons are shared between atoms, such that the electrons are attracted to two nuclei. Non-metal atoms will form covalent bonds with each other. In Model 2a, two fluorine atoms form a stable F2 molecule in which each atom has an octet of valence electrons by sharing a pair of electrons. Notice in Model 2b that the molecules themselves are not connected by covalent bonds to oneanother, but that the atoms are connected into small units, called molecules, by covalent bonds. Thus, molecular covalent substances consist of a large group of individual molecules. Note that whenever we are talking about molecules in chemistry, we are referring to covalently bonded groups of atoms. Molecular covalent substances tend to be liquids, gases or soft solids. This is because the individual molecules have more freedom to move within the substance. Covalently bonded atoms can share two pairs of electrons (double bond) or three pairs of electrons (triple bond). For instance, oxygen in air does not exist as a single atom. Model 3 shows two oxygen atoms sharing 2 valence electrons each to form O2. All the macromolecules that comprise the human body (DNA, carbohydrates, lipids, and proteins) are covalently bonded. Model 3b shows a typical saturated and unsaturated fat molecule. Covalently bonded molecules can form very large and complex molecular structures because of all the combinations and bonds that can be made to a few central atoms such as carbon.In fact, organic chemistry is an entire branch of chemistry that focuses solely on these large, carbon based molecules (and is a dreaded class taken by most science majors).
III. Metallic Bonding: Model 4 is referred to as metallic bonding. In the metallic bond, metal atoms achieve a more stable configuration by sharing the electrons in its outer shell with many other metal atoms. The valence electrons in metals are not tightly bound in the nucleus and each atom in a metal contributes all the electrons in its valence shell to all other atoms in the structure. The electrons then form a sort of “sea of electrons” around the atoms. Thus, each atom becomes essentially positive in charge, having lost some electrons. The atoms in turn are attracted to the negatively charged "sea". Therefore, the individual atoms can "slip" over one another yet remain firmly held together by the electrostatic forces exerted by the electrons. This is why most metalscan be hammered into thin sheets (malleable) or drawn into thin wires (ductile). When electricity is applied, the electrons move freely between atoms, and a current flows. In Model 4b is an artist's rendering of a block of iron, Fe, atoms. The "sea of electrons" is notvisible.















Useful 👍🏻
ReplyDelete❣️❣️thnks
ReplyDeleteits useful thank you
ReplyDelete